NCERT Class 7 Science Sixth Chapter Physical and Chemical Changes Exercise Solution
Physical and Chemical Changes
Inside Questions with Answers:
Define physical properties.
Ans. Properties such as shape, size, colour and state of a substance are called its physical properties.
What is called physical change?
Ans. A change in which a substance undergoes a change in its physical properties is called a physical change.
What is called rusting?
Ans. If we leave a piece of iron in the open for some time, it acquires a film of brownish substance. This substance is called rust and the process is called rusting.
What is called chemical change?
Ans. A change in which one or more new substances are formed is called a chemical change.
How do we prevent rusting?
Ans. Prevent iron articles from coming in contact with oxygen, or water, or both. One simple way is to apply a coat of paint or grease. In fact, these coats should be applied regularly to prevent rusting. Another way is to deposit a layer of a metal like chromium or zinc on iron. This process of depositing a layer of zinc on iron is called galvanisation.
What is called crystallization?
Ans. Large crystals of pure substances can be formed from their solutions. The process is called crystallisation.
What types of changes can we see in Chemical Changes?
Ans. Heat, light or any other radiation (ultraviolet, for example) may be given off or absorbed.
Sound may be produced.
A change in smell may take place
a new smell may be given off.
A colour change may take place .
A gas may be formed.
Give some Example of Chemical Change.
Ans. digestion of food in our body, ripening of fruits, fermentation of grapes, etc.,
Which layer acts as a natural shield against this radiation.
Ans Ozone Layer.
Define Stainless steel does not rust.
Ans. Stainless steel is made by mixing iron with carbon and metals like chromium, nickel and manganese. It does not rust.
Exercise Questions with Answers:
(1) Classify the changes involved in the following processes as physical or chemical changes:
(a) Photosynthesis
(b) Dissolving sugar in water
(c) Burning of coal
(d) Melting of wax
(e) Beating aluminium to make aluminium foil
(f ) Digestion of food
Ans:
(a) Photosynthesis – Chemical Changes.
(b) Dissolving sugar in water – Physical Changes.
(c) Burning of coal – Chemical Changes.
(d) Melting of wax – Physical Changes.
(e) Beating aluminium to make aluminium foil – Physical Changes.
(f ) Digestion of food – Chemical Changes.
(2) State whether the following statements are true or false. In case a statement is false, write the corrected statement in your notebook.
(a) Cutting a log of wood into pieces is a chemical change. (True/False)
Ans. False. It’s a physical change because there is no new substances are formed.
(b) Formation of manure from leaves is a physical change. (True/False)
Ans. False. It’s a chemical change because there is new substance is formed.
(c) Iron pipes coated with zinc do not get rusted easily. (True/False)
Ans. True.
(d) Iron and rust are the same substances. (True/False) .
Ans. False. They are the different substances.
(e) Condensation of steam is not a chemical change. (True/False)
Ans. True.
(3) Fill in the blanks in the following statements:
(a) When carbon dioxide is passed through lime water, it turns milky due to the formation of _________.
(b) The chemical name of baking soda is _________.
(c) Two methods by which rusting of iron can be prevented are _________ and _________.
(d) Changes in which only _________ properties of a substance change are called physical changes.
(e) Changes in which new substances are formed are called _________ changes
Ans. (a) calcium carbonate
(b) sodium hydrogen carbonate
(c) painting or greasing, galvanisation
(d) physical
(e) chemical
(4) When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas. What type of change is it? Explain.
Ans. It’s a chemical change. Because when baking soda is mixed with lemon juice there is new substances are formed.
Lemon Juice (Citric Acid) + Baking soda > Bubbles (Co2) + Other Substance.
(5) When a candle burns, both physical and chemical changes take place. Identify these changes. Give another example of a familiar process in which both the chemical and physical changes take place.
Physical change when a candle burns: Melting of wax, Vapourisation of melted wax.
Chemical change when a candle burns: Burning of vapours of wax to give carbon dioxide, heat and light.
Liquid Petroleum Gas (LPG) is an another example of a familiar process in which both the chemical and physical changes take place.
(6) How would you show that setting of curd is a chemical change?
Ans. Curd is made from milk. When curd is made from milk therefore milk can not be reversible so that we can easily tell that it’s a chemical change. Another proved example are following that setting of curd is a chemical change-
(I) A change in smell may take place.
(II) A colour change may take place .
(7) Explain why burning of wood and cutting it into small pieces are considered as two different types of changes.
Ans. Burning of wood is a chemical change because it cannot be transformed is its original formed whoever cutting it into small pieces are considered as physical change cause there are no new substance are formed.
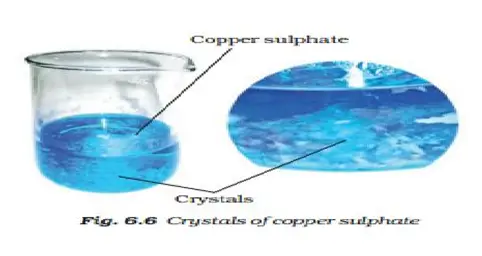
(8) Describe how crystals of copper sulphate are prepared.
Take a cupful of water in a beaker and add a few drops of dilute sulphuric acid. Heat the water. When it starts boiling add copper sulphate powder slowly while stirring continuously. Continue adding copper sulphate powder till no more powder can be dissolved. Filter the solution. Allow it to cool. Do not disturb the solution when it is cooling. Look at the solution after some time. Can you see the crystals of copper sulphate? If not, wait for some more time.
(9) Explain how painting of an iron gate prevents it from rusting.
Ans. Painting of an iron gate prevents it from rusting here the details in following-
Ships are made of iron and a part of them remains under water. On the part above water also, water drops keep clinging to the ship’s outer surface. Moreover, the water of the sea contains many salts. The salt water makes the process of rust formation faster. Therefore, ships suffer a lot of damage from rusting in spite of being painted.
(10) Explain why rusting of iron objects is faster in coastal areas than in deserts.
Ans. We know that the presence of both oxygen and water (or water vapour) is essential for rusting of iron. In coastal areas rusting of iron objects is faster because thepresence of both oxygen and water (or water vapour) is higher than the desert areas.
(11) The gas we use in the kitchen is called liquified petroleum gas (LPG). In the cylinder it exist as a liquid. When it comes out from the cylinder it becomes a gas (Change – A) then it burns (Change – B). The following statements pertain to these changes. Choose the correct one.
(i) Process – A is a chemical change.
(ii) Process – B is a chemical change.
(iii) Both processes A and B are chemical changes.
(iv) None of these processes is a chemical change
Ans. (ii) Process – B is a chemical change.
(12) Anaerobic bacteria digest animal waste and produce biogas (Change – A). The biogas is then burnt as fuel (Change – B). The following statements pertain to these changes. Choose the correct one.
(i) Process – A is a chemical change.
(ii) Process – B is a chemical change
(iii) Both processes A and B are chemical changes.
(iv) None of these processes is a chemical change.
Ans. (iii) Both processes A and B are chemical changes.
Leave a Reply